What Is the Noble Gas Configuration for Strontium
When light beams are. What is the noble gas configuration for strontium Sr.

Electron Configuration Ppt Video Online Download
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
. Its electronic configuration is Kr5s 2. In a similar fashion strontium has two more electrons than the noble. SRO is formed by two elements ie.
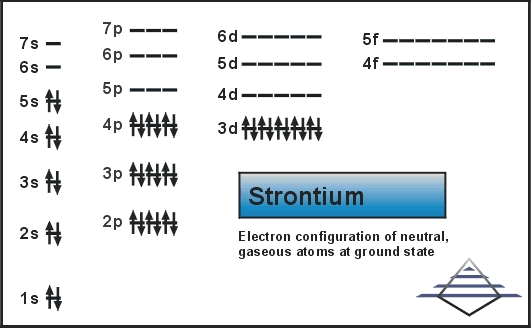
Electron configuration of Strontium is Kr 5s2. Possible oxidation states are 2. Therefore the number of electrons in.
The noble gas configuration of strontium is Kr5s2. Strontium has atomic number 38. 2 Names and Identifiers.
The noble gas configuration of this element is Kr. Highlight the blank areas to reveal the answers electron. What is the noble gas configuration of strontium.
1 Structures Expand this section. Strontium2 is a strontium cation a divalent metal cation and a monoatomic dication. It has a role as a cofactor.
The noble gas configuration for silicon is. What is the molecular formula. Ne3s 2 3p 2.
One is strontium and other is oxygen. Potassium has nineteen electrons one more than the noble gas argon so its configuration could be written as Ar4s 1. Electrons and Electron Configuration.
The electron configuration for strontium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2 according to the Jefferson Lab website. Next we shall determine the electronic configuration of the noble gas element before strontium Sr. When it losses two electron from 5s orbital.
Express your answer as a chemical formula. The molar mass of the compound was found to be 30. Atomic number of strontium Sr 38.
Electron Configuration and Oxidation States of Strontium.

Strontium Electron Configuration Sr With Orbital Diagram

Strontium Electron Configuration Sr With Orbital Diagram

How To Write The Electron Configuration For Strontium Sr Youtube

How To Write The Electron Configuration For The Sr 2 Strontium Ion Youtube
No comments for "What Is the Noble Gas Configuration for Strontium"
Post a Comment